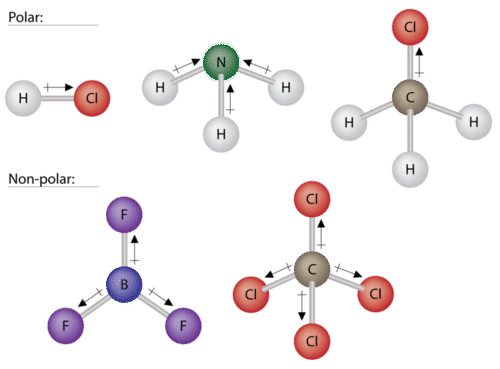
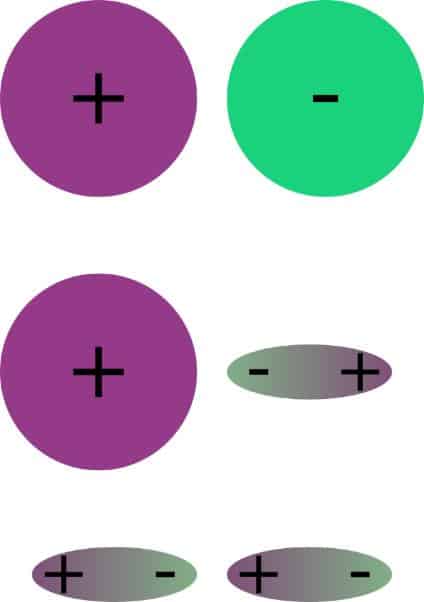
SOLVED: Which of the: substances has polar interactions (dipole-dipole forces) between molecules? CHCI, I,O

3D Interaction Homology: Hydropathic Analyses of the “π–Cation” and “π–π” Interaction Motifs in Phenylalanine, Tyrosine, and Tryptophan Residues | Journal of Chemical Information and Modeling

Destabilization of polar interactions in the prion protein triggers misfolding and oligomerization - Bhate - 2021 - Protein Science - Wiley Online Library

Ion-induced oil–water wettability alteration of rock surfaces. Part I: Polar interactions between oil and solid - ScienceDirect

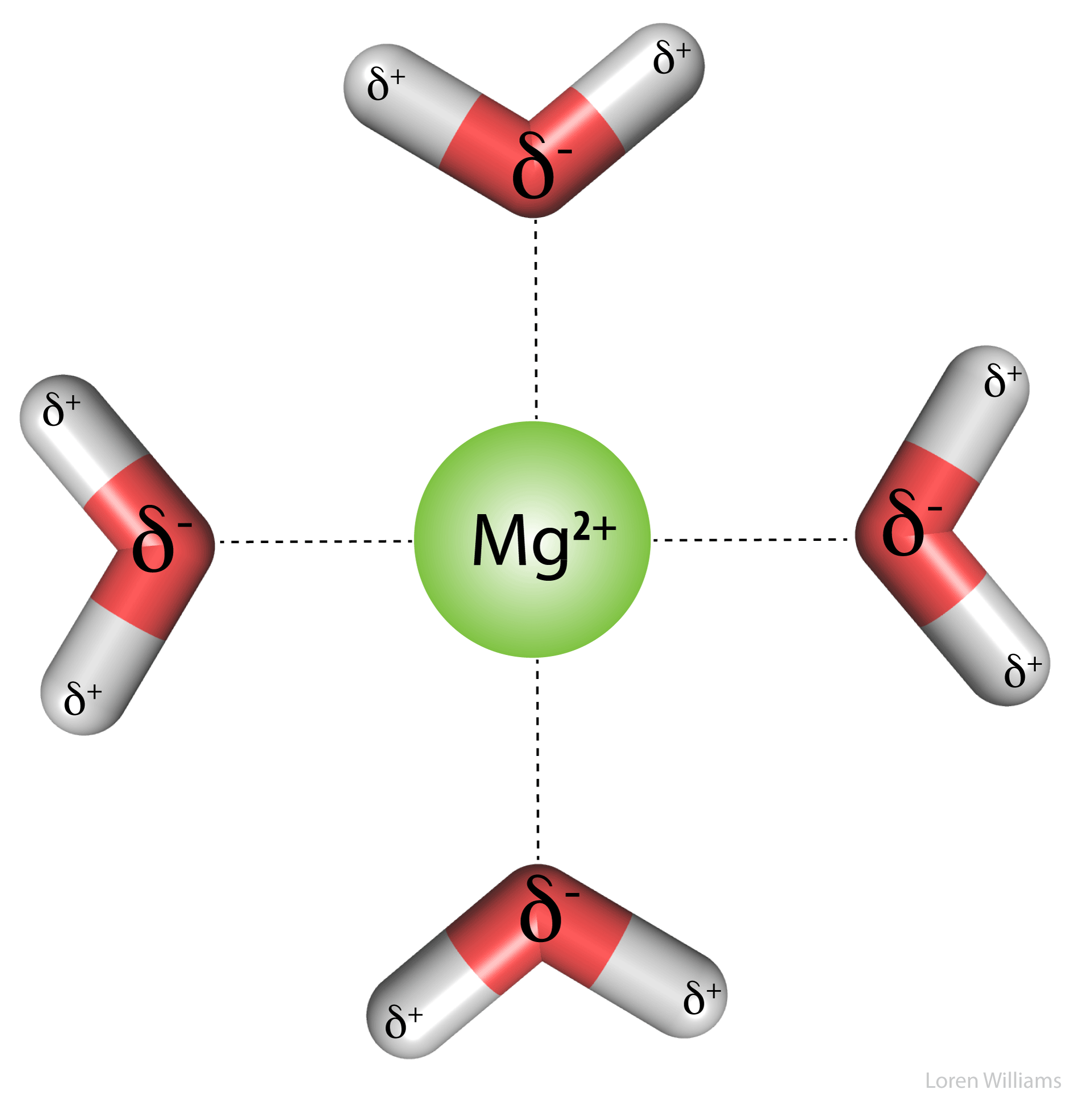
Representation of coordinated water molecules and polar interactions... | Download Scientific Diagram

organic chemistry - If all intermolecular forces are electrostatic in nature, why don't large non polar molecule dissolve in water? - Chemistry Stack Exchange
On the Importance of Polar Interactions for Complexes Containing Intrinsically Disordered Proteins | PLOS Computational Biology